Compare all Pack Sizes
Unfortunately, we are not currently accepting orders for Northern Ireland, Jersey and Guernsey.
We can only ship to the following postcodes where a Royal Mail postal option has been chosen at checkout.
AB, DD, DG3, DG4, DG6 - DG9, EH, FK, G, HS, IM, IV, KA, KW, KY, ML, PA, PH, PO30 - PO41, TD, TR21 - TR25, ZE
What is Silver Nitrate? It is an inorganic compound, chemical formula AgNO₃, that consists of silver, nitrogen, and oxygen. It is a colourless, crystalline substance that is highly soluble in water and is widely used in various chemical, industrial, and laboratory applications. Silver Nitrate is also known by alternate names such as Lunar caustic, Silver(I) nitrate, or Argentum nitrate.
Consistent Quality: We source only high-quality Silver Nitrate, meeting the standards of ACS, ensuring reliable and consistent performance in laboratory, industrial, and chemical applications.
Shipped from Manchester, UK Facility: Sourced and packaged in non-toxic, chemical-resistant containers to ensure safe handling and maintain product quality during transport.
Photography
Silver Nitrate has historically been essential in photographic processes. It is used in the preparation of photographic emulsions for film, paper, and plates, where it reacts with light to create images. Despite the shift to digital technology, it remains a key component in traditional photography.
Chemical Synthesis
Used in organic and inorganic chemical synthesis, including the preparation of silver salts, dyes, and other compounds. It is involved in a variety of reactions, particularly in the production of chemicals for the electronics, jewellery, and chemical industries.
Electronics and Plating
Plays a crucial role in electroplating and the manufacture of electronic components. It is used to deposit a thin layer of silver on various materials, improving conductivity and corrosion resistance in electronic devices and components.
Laboratory and Educational Uses
Frequently used in educational settings and laboratories for experiments involving chemical reactions, particularly those related to halides, precipitates, and redox reactions.
Minimum Assay: >99.9%
Molecular Formula: AgNO3
Molecular Weight: 169.87 g/mol
Melting Point: 212 °C
Chloride (Cl): <1ppm
Sulphate (SO4): <5ppm
Copper (Cu): <0.1ppm
Iron (Fe): <0.2ppm
Lead (Pb): <1ppm
Packing Group: 2
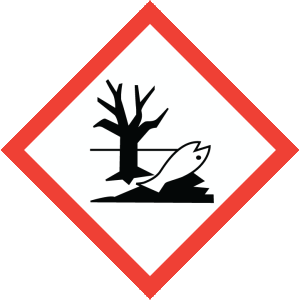
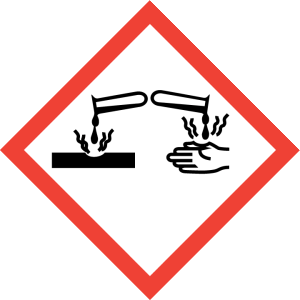
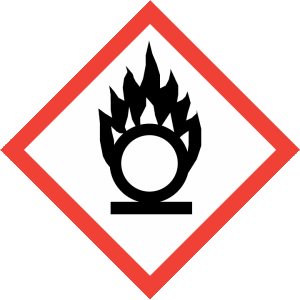
Hazard Phrases: May intensify fire; oxidiser. May be corrosive to metals. Causes severe skin burns and eye damage. Very toxic to aquatic life with long lasting effects.
Prec Phrases: Keep away from heat/sparks/open flames/hot surfaces. Keep/Store away from clothing/ Do not breathe dust/fume/gas/mist/vapours/spray. Wear protective gloves/protective clothing/eye protection/face protection. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. In case of fire: Use


We can also accept a bank transfer (UK Only)